So I now have two-third of the metal coil of the heater submerged into the water in a plastic jug, ready to prepare a cup of coffee. I need just a cup, not the whole volume of water in the jug. It’s getting complicated; I need the water faster to get me reading and sipping. When will the full jug of water get hot? Hmmm. Can science answer this?
I was silent for a while, thus I was not talking but building a set of thoughts and imagination about how to get my cup of hot water before the whole jug of water gets hot.
Water is deliberately made up of tiny independent and self existent units called molecules. Each water molecule carries within itself two ions of hydrogen bonded to an ion of oxygen. The convection currents according to the thermodynamics of a fluid explain: when heating fluids, molecules closer to the source of heat attain a higher energy which causes their acceleration and displacement from their original position of random vibrational motion, just as the reverse will do the opposite (thus a moving particle generates heat directly proportional to its momentum). When the water molecules attain a higher temperature, or perhaps absorb much heat energy, the molecules expand and bear a less density. The expansion should be as a result of weakness of the H-O-H bond, causing each hydrogen ion to radiate farther from the oxygen ion as compared to its state at room temperature.
A bit of chemistry here, but physics takes over again. The expanded molecules, having a lesser density can not remain at the same depth with the fairly cold molecules; rather they will have to float over the cold molecules (a body will float when its density is lesser than the density of the fluid in which it is placed—principle of floatation). So in time I should have the water just below the surface getting hot before the molecules at the bottom.
I expected my pondering to come up true but it was four minutes already, and the water only fairly warm.
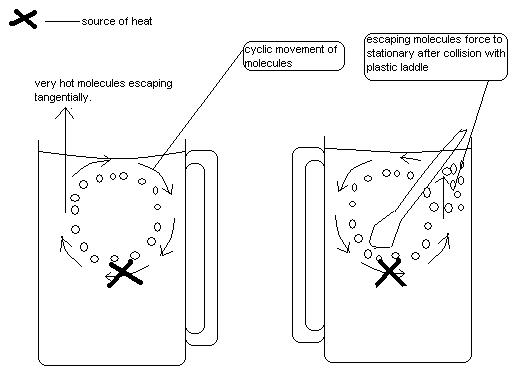
Then it hit me; if the molecules around the heater gain energy and bounce off to the surface, then the molecules very close to the heater should attain greater energy which may affect my expectation. If I’m true, then those molecules might be escaping from the surface carrying along with them all the heat they absorbed. The cyclic convectional currents are causing the movements of the energetic water molecules to help heat my water, but again they are causing the very heated molecules I needed for my coffee to escape. As the currents are cyclic, hence in a vertical circular shape, the escape of those very energetic molecules might be tangential.
Then it hit me; if the molecules around the heater gain energy and bounce off to the surface, then the molecules very close to the heater should attain greater energy which may affect my expectation. If I’m true, then those molecules might be escaping from the surface carrying along with them all the heat they absorbed. The cyclic convectional currents are causing the movements of the energetic water molecules to help heat my water, but again they are causing the very heated molecules I needed for my coffee to escape. As the currents are cyclic, hence in a vertical circular shape, the escape of those very energetic molecules might be tangential.
May be I should just place a plastic spoon or something else into the water in order to block the currents. “After this, the molecules will just ascend like a helicopter does, to the surface without escaping tangentially in their cyclic movement”, I thought.
How do I know where exactly to place this plastic material and what will be its mechanism to help manifest what I was thinking?
First of all the spoon will also absorb some heat energy, but its plastic nature makes the absorption minimal, I smiled. Secondly it will disrupt the movement of the molecules to avoid their escape by colliding with them to reduce their speed (this can be explained by relative motion between two bodies). When a body moves toward a stationary body, it loses some of its kinetic energy to the stationary body during the collision. Other times it gains more energy or bounces back with the same velocity and hence same energy. Molecules rarely undergo perfectly elastic collision, meaning when a molecule collides with another molecule or body, it bounces back with a different velocity and energy. This will conclude that, when the very hot water molecules collide with the ladle, they will lose some velocity enough to keep them stationary.
This is great; soon I will have my hot coffee. I immersed a plastic ladle into the water to cause the effect I discussed.
Unfortunately it was already two minutes late and nothing I thought had happened. Oh yes! I remembered the uncertainty principle in quantum mechanics which explains: the position and momentum of a particle can never be said to be known accurately. Meaning one can’t tell the displacement of a particle accurately and to determine its speed. Immersing the ladle into the water means nothing here; I could not identify the molecules, their momentum and speed as they moved in the convectional currents. They might have had a different direction which avoided the collision I expected, and also a lesser speed which can only cause very little amount of kinetic energy lost or may be even accelerated with greater energy after the collision.
Science is fun and practical, but without the pleasure to be curious and conscious in all matters of the nature around us, science oxidizes gradually into a rusted scrap. The methods used in science can be explained plainly for the illiterate to comprehend.
Solving a problem in science is like three men throwing stones to plug a ripe mango from a tall tree.
Let’s name these men A, B, and C and imagine that A is very tall, B is averagely tall but C is very short.
All three have an aim to plug the mango. This aim can be translated physically to mean their common angle of elevation but different projectiles for each person’s throw. Since the stalk is thin and lighter than the stones to be thrown, the reaction force exerted by the stalk is negligible, making the stalk the best point to hit the mango to get it falling. The fruit is rather large, and may have to take three to four hits before getting plugged off the tree. The curves show the possible projectiles to be observed..
From the curves, it can be deduced that C will require a lot of energy to fly his stone through the long curve, B requires moderate energy as A requires the least. Viewing another point, we will find out that A’s throw will have the greatest repulsion from gravitational influence. This is because A’s throw has the steepest gradient.
The conclusion each of them wants is the mango falling off when hit by their stones.
All the brainstorming only explains science to be an art of solving problems by using problems. Whenever there is a scientific mystery, there are a lot of ways to solve the problems, not only one identified and studied method. As in the above scenario, each person has his personal difficulties to overcome for a common goal. But if the short thrower considers the position the tallest makes to the three, he will manage to calculate the best position he needs for the best hit.
Science and its methods, principles, laws and theories are just like these three throwers, they keep depending on each other to help explain and solve a problem.
In 1900, Max Planck was working on the curve of radiation wavelengths at various temperatures. He came up with an equation which had an odd feature. The equation required a tiny quantity needed to be included for it to come up right. Later the tiny quantity was called Planck’s constant, and its one of the world’s known fundamental constants. Max thought his mathematical experiment and equation had no physical effect to the nature. He came up that the equation affected not the fundamental nature of light but action of light when absorbed or emitted by a body. The conclusion drawn was that energy is composed of definite number of equal finite packages. Planck left a mystery; the Plank’s constant –h.
There was also a dazzling topic at the moment, the photoelectric effect, which caused electrons to be knocked off the surface of a metal when light is incident on the metal. In Philip Lenard’s experiment on the photoelectric effect, he observed that when he increased the frequency of the light, the electrons emitted with great energy. He decided to rather increase the intensity of the light. This will produce light rays with a lot of energy to knock off electrons with greater energy. This never happened; rather a greater number of electrons were produced with no change in their energy. He kept increasing the intensity of the light but nothing happened. The problem he left was the reason change in intensity of light had no effect on emitted electrons.
Four years had wiped away, and Einstein pondered over the works of Lenard and Planck. It was 1904 when Einstein finished his work on the general molecular theory of heat. He treated black body radiations as if they were particles, as gases contain molecules. Einstein compared the change in enthalpy of a gas when its volume changes to the change in enthalpy when the volume of a black body radiation changed. The rate of enthalpy change in black body radiation was just as that in an ideal gas. So Einstein would conclude that the black body radiations were made of tiny particles just as a gas was made of molecules. He compared the work of Lenard and Planck to his work
Einstein would explain that the energy of an electron emitted is proportional to its frequency. The energy of the emitted electron therefore would be given by the product of Planck’s constant and the frequency of light causing the emission. (E = hf)
Increasing the intensity of light will mean increasing the number of light photons from the source of light, and each photon or packet of light energy will knock a definite number of electrons like other photons.
So Einstein took two problems from others and created a solution—science.
I gave up all the science in my cup of coffee, covered the jug partially with a lid.
I started reading as I waited patiently for the water to get hot. Surprisingly it began to boil in two minutes which I less expected. Could it have been the cause of the lid?
Oh yes?
The lid should have been preventing the escape of the energetic molecules, causing them to return into the water. The other molecules that remained over the surface and below the lid caused an increase in the vapor pressure exerted on the water. Therefore the water boiled earlier because the vapor pressure counterbalanced atmospheric pressure...
Science is an amalgamation of all aspects of nature. If a scientific idea fails to reflect in other aspects or can’t be explained using already existing ideas, it’s almost true to be untrue as a scientific thought… my little cup of coffee is driven me to think of all the possibility of science in its preparation. If I want to continue about how the coffee and sugar dissolved, and how my taste buds sensed its taste, and how I could smell the blackness of the coffee in my nostrils, I will leave too many problems for you to solve.
Solve my mistakes in preparing my coffee and I prefer you don’t mind a sip.
You are invited.



THIS IS ABSOLUTE PIECE OF GENIUS TUMBS UP TO YOU GUYS
ReplyDeleteawesome ,this is really sick stuff!!!!!!!!!!
ReplyDeleteBrilliant article, keep it up!
ReplyDelete